8 min read
Modeling the Impact of SARS-CoV-2 Omicron Variant Mutations
On November 24, 2021, South Africa reported the identification of a new SARS-CoV-2 variant, B.1.1.529, to the World Health Organization (WHO). On November 26, 2021, the WHO designated B.1.1.529 as a variant of concern (VOC) named Omicron. This new variant features a number of concerning spike protein mutations. As illustrated below, these key mutations, also found in earlier variants, are present at or near sites regulating virus infectivity. In addition, Omicron displays a surprising number of new mutations. Of special interest are mutations occurring in the RBD and S1/S2 domain. The RBD is important for binding to cells, and the S1/S2 is important for processing the virus to enable entry into the cells.
Spike Mutations of Interest Found in SARS-CoV-2 Variants of Concern
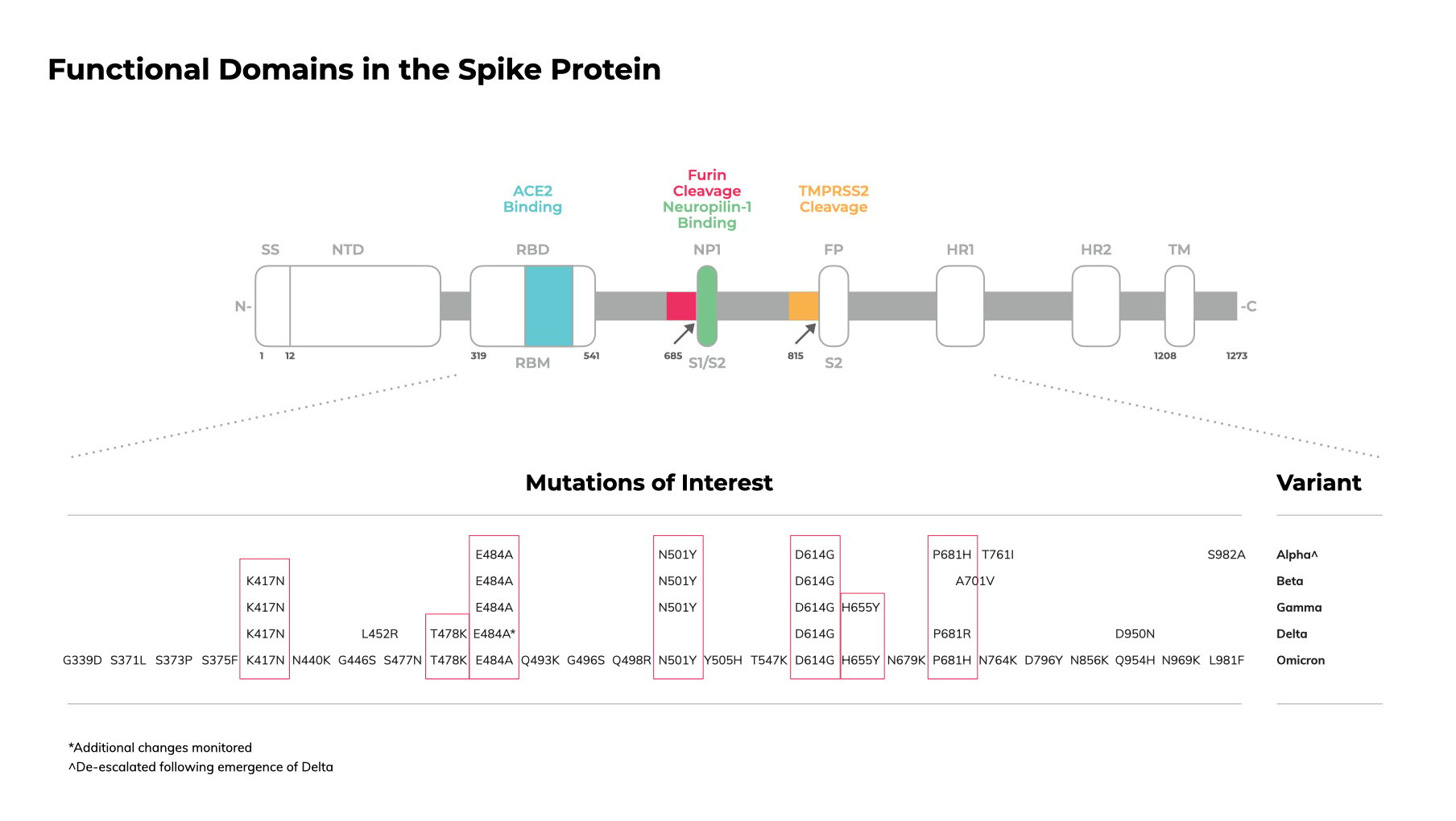
The upper figure represents spike protein monomer and key domains regulating infectivity. The lower figure shows spike mutations of interest and includes changes within residues 319-541 (receptor binding domain), 613-705 (S1/S2 furin cleavage domain) and a small stretch of residues n-terminal to 815 (S2’ TMPRSS2 cleavage domain). Additional mutations specific to Omicron are included. Boxes indicate mutations common across the variants. Not all spike protein amino acid changes are included.
It is still unclear how the new mutations in Omicron may impact transmission, infection, immune escape or protection by available vaccines and monoclonal antibody therapeutics. Laboratory studies are expected to take weeks (or months) to answer these unknowns. In this blog, we describe the results of modeling studies of functional domains and the impact Omicron mutations may have on infectivity. Additionally, we discuss mutational analysis by others and their potential impact upon immune escape and protection.
Forecasting Omicron infectivity through molecular modeling
In previous blog posts, we have shown how we use our suite of molecular modeling tools to predict the impact of spike protein mutations on the functional activity of the receptor binding domain (RBD) and the S1/S2 cleavage site. Using these tools, Antibody Solutions was one of the first to propose that the earliest variant, D614G, was more transmissible because it promoted furin processing, a key step in virus infection. We found that the D614G mutation induces a conformational change in the S1/S2 site that arranges the R4 arginine (R682) closer to serine 686, which favors cleavage by furin. Additionally, mutations in the S1/S2 site of Alpha and especially Delta further favor cleavage of the S1/S2 site by furin.
We performed protein docking analysis incorporating mutated residues of the variants into models generated from the crystal structure of the original SARS-CoV-2 spike protein RBD bound to ACE2. Energy differences (ΔE) were calculated by minimizing the energy of the variant structure and comparing it to the original structure. For the Alpha variant, we observed two mutations in the RBD, E484K and N501Y, that energetically favored the interaction of the RBD with the ACE2 receptor. By contrast, in Delta the L452R and T478K mutations were less energetically favored.
Our observations argue that the superior fitness of Delta over previous variants is conferred by mutations in the S1/S2 site versus the RBD. Our hypothesis is supported by the findings of others that: (a) furin more efficiently cleaves Delta at the S1/S2 site compared to either the Alpha or wild type virus; and (b) Delta has a lower affinity for ACE2 compared to Alpha, as predicted by our modeling.
What are the implications of Omicron mutations on spike protein function?
As illustrated in the figure Spike Mutations of Interest Found in SARS-CoV-2 Variants of Concern (above), Omicron shows several mutations in the RBD and the S1/S2 furin cleavage site, with many similar to those found in the Alpha and Delta variants.
RBD binding to ACE2
In the RBD, Omicron has multiple mutations that energetically favor formation of the RBD/ACE2 complex. The mutations E484A and N501Y lower the energy required for complex formation (-1.02 and -1.35, respectively), just as they have done in other variants. Additionally, Omicron has mutations S375F, S371L and G339D that further improve stability of the complex. We found that all of the mutations in Omicron lower the energy required for complex formation to ΔE -3.95 when compared to the original Wuhan RBD. This is more favorable than Alpha (ΔE -2.14) or Delta (ΔE 1.6). The combined mutations would be expected to increase binding affinity for the Omicron spike protein to ACE2, which could increase infectivity.
Mutations in the Omicron RBD favor association of the spike protein with ACE2
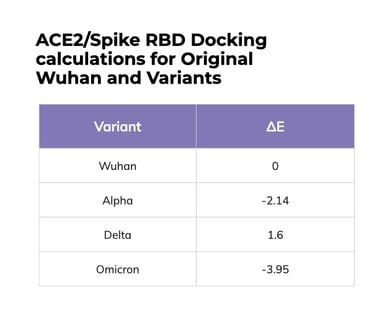
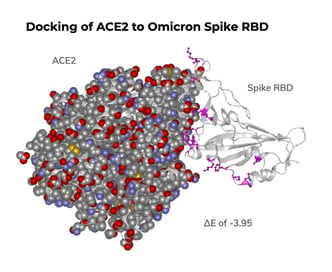
Protein docking analysis was performed by incorporating mutated residues of the variants into models generated from the crystal structure of the original SARS-CoV-2 spike protein RBD bound to ACE2. ΔE were calculated by minimizing the energy of the variant structure and comparing it to the original structure. The 15 RBD mutations are colored magenta: G339D, S371L, S373P, S375F, K417N, N440K, G446S, S477N, T478K, E484A, Q493K, G496S, Q498R, N501Y, Y505H.
Furin cleavage of the S1/S2 site
Mutations at the furin cleavage site, both new and pre-existing, afford insight into the transmissibility and infectivity of the Omicron variant. Omicron features an H655Y mutation, previously seen in the Gamma variant, that is proximal to the furin cleavage site. P681H, which has appeared in both Alpha and Omicron, has been shown to enhance spike cleavage, which could aid transmission. This mutation is found both in Alpha and, in an alternate mutation at this position (P681R), in Delta.
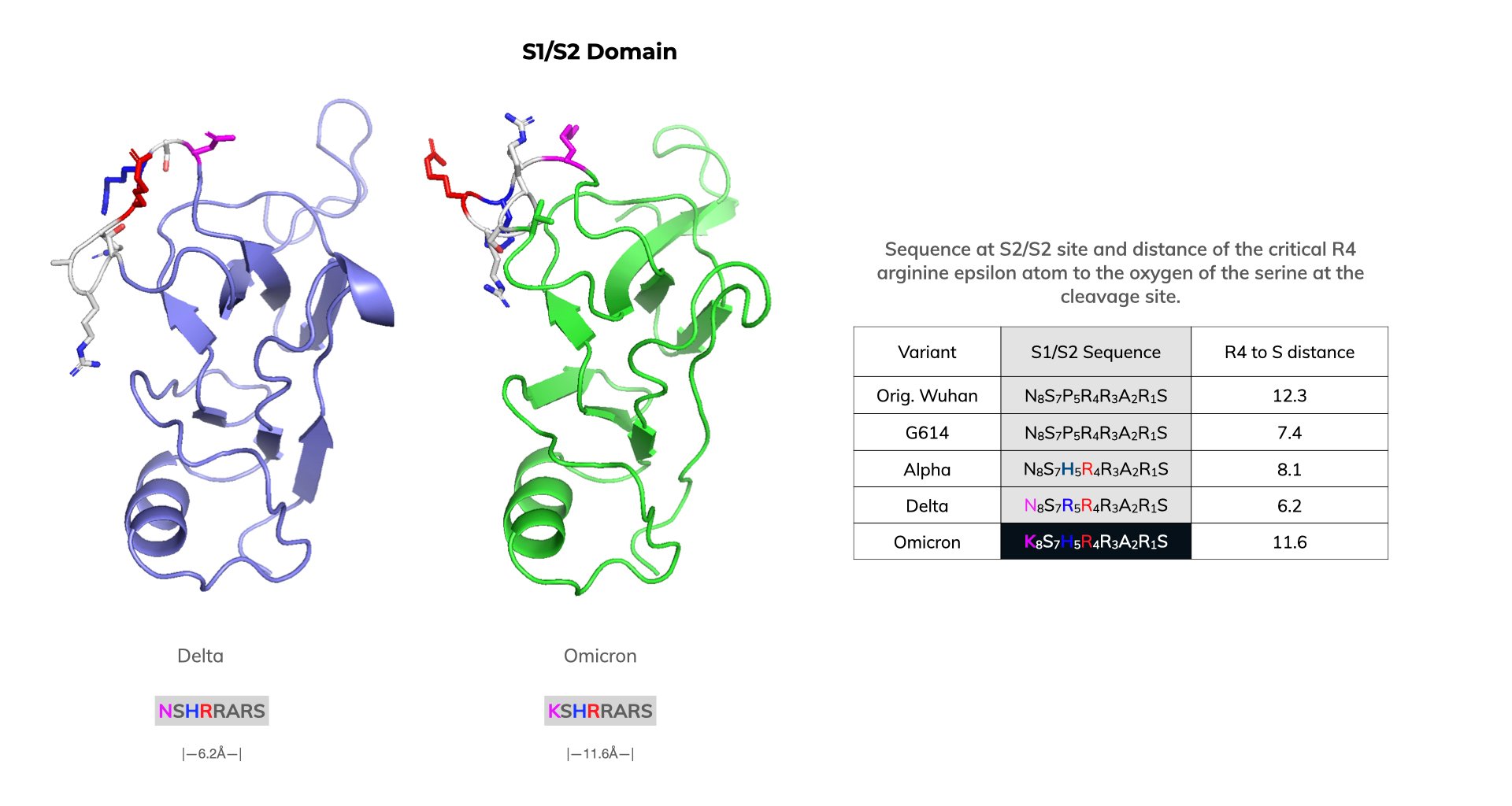
N679K is another mutation that is proximal to the S1/S2 furin cleavage site. This mutation adds to the polybasic nature of the site and, theoretically, could increase spike cleavage. However, in our modeling, we found the N679K mutation increased the distance from the critical R4 arginine epsilon atom to the oxygen of the serine at the cleavage site to 11.6 ångströms (Å). This distance is similar to that found in the original Wuhan virus (12.3 Å). One would anticipate that the presence of N679K in the S1/S2 domain would make it less favorable for furin cleavage. By contrast, the Delta variant has the shortest distance (6.2 Å) from R4 to S. What’s more, it contains an added arginine at position R5 with a distance to S of 8.3 Å. Both the closer orientation of the R4 arginine and additional R5 arginine proximal to the catalytic site would be predicted to more effectively promote furin cleavage compared to other variants.
Orientation of Key Residues for Furin in the S1/S2 Domain Are Less Favorable in Omicron
Region 590-710 SARS-CoV-2 spike proteins. All models aligned using Pymol. Color coding: Ser in orange, critical arginines R4 for catalysis in red, position 5 amino acid in blue, and position 8 amino acid in magenta. Distances in ångströms (Å) between the epsilon atom of the arginine in the R4 or R5 positions and the side chain oxygen of the Serine (S) at the cleavage site are indicated.
What will be the impact on transmissibility vs. infectivity?
Overall, the lower free energy of Omicron for ACE2 spike protein complex formation gives it an advantage over Delta for binding to ACE2 expressing cells. However, the more favorable conformation of the Delta S1/S2 site would give Delta the advantage for processing the spike protein that is required for infecting cells.
If transmissibility is the capacity of a pathogen to pass from one organism to another, and infectivity is the ability of a pathogen to establish an infection, could one variant be more favored for transmissibility and the other for infectivity? Omicron’s mutations in the RBD would favor binding to ACE2, while Delta’s mutations in the S1/S2 site would favor processing by the host protease furin and, ultimately, transport into the cell. Based upon these distinct mechanisms, Omicron might be more transmissible but Delta more infective. Whether this turns out to be the case, and how this will impact our world at large, still remains to be seen.
Is Omicron more or less immunogenic?
A report from Politecnico di Milano (Bernasconi, et al) provides an in-depth look at the effect of mutations in Omicron spike protein on predicted T- and B-cell binding sites or epitopes. A higher number of T- and B-cell epitopes would increase the immunogenicity of the variant. Compared to Delta, the Omicron variant has a larger number of T-cell epitopes (27% vs. 8.4%) and B-cell epitopes (31% to 11%). Thus, the Omicron variant mutations would not appear to aid the virus in avoiding an immune response.
Will vaccines or previous infections provide immunity?
The large number of Omicron mutations within potential antibody epitopes would likely impact the protection afforded by existing vaccines. Some antibodies raised to pre-variant infections or vaccines would have lower binding activity to Omicron. As illustrated in our model of the spike RBD, many of the mutations are in exposed positions that are likely to be easy antibody targets. The E484X mutation, in Omicron and other variants, has been found to be a critical target of antibodies against SARS-CoV-2. Measurements of the ability of sera, either from vaccinated persons or individuals with previous SARS-CoV-2 infections, to neutralize Omicron should answer this question soon.
Will existing monoclonal antibody treatments work?
There is currently no virus-specific data available to assess whether monoclonal antibody treatments will have efficacy against the Omicron variant. Based on data from other variants with significantly fewer changes in the RBD, the Omicron variant is expected to remain susceptible to some monoclonal antibody treatments, while others will display less potency. Single mutations including K417N, N440K, S477N, T478K, Q498R and N501Y have been found to lower neutralization titers of monoclonal antibody therapeutics from Eli Lily, Regeneron and GSK/Vir.
How worried should we be about Omicron’s mutations?
Our analysis of the changes in the spike protein of Omicron suggests a mixed bag of effects. It appears to be better for cell binding but less favorable for processing. It’s interesting to speculate that the Omicron variant may be more transmissible through better binding to ACE2, but less infective due to less efficient cleavage by furin. While prior infections and vaccines may be less effective, the increased number of T- and B-cell epitopes would make Omicron a better target for our natural immune response. There is still a need for therapeutic antibodies whose binding is not affected by Omicron mutations and that target more than one domain regulating infectivity.
Time will tell. We will continue to publish additional findings from our SARS-CoV-2 research, and we encourage you to please let us know what you are working on and if we can support your efforts with our technology and expertise. You can get in touch with us here.

Author of more than 40 publications, John’s current research interests include new technologies for improving therapeutic antibody discovery, properties of next-generation antibody-like molecules, and best practices for critical reagents used in biologics development.